Premium Dry Shippers for Cryogenic Shipping
Imagine a life-saving cancer treatment, meticulously developed in a state-of-the-art laboratory, losing its potency from a handling error while loading or unloading it into its dry shipper. This is a daily risk for many cryogenic shipments.
Aside from supporting clinical trials processes, cryo-shipping supports healthcare, pharmaceuticals, biotechnology and other life sciences sectors. To add, this shipping method is key in the progression of regenerative medicines and cell & gene therapy as they maintain the viability of the cells and tissue for potential medical breakthroughs. According to Grand View Research, the cryogenic equipment market size will grow by 55.82% from 2023 to 2030.
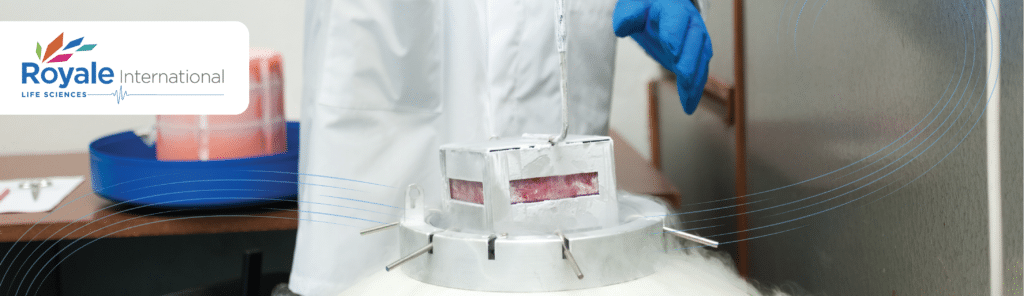
Thus, dry (vapor) shippers have become necessary to support cryo-shipping supply chains. These specially designed containers, insulated by liquid nitrogen, ensure the safe transport of large collections of biological material, preserving their integrity and safeguarding both users and transporters.
—————- A brief introduction: “Dry Shipper 101” —————-–
What challenges turn up the heat for dry shippers?
There are three key P’s that may challenge cryogenic shipping procedures –
Product –
Whether it be embryos, organs or tissue samples, these products need to be kept between -196°C to -150°C to stay viable. Moreover, cryo-shipments can vary in size, ranging from a single organ to racks of vials. It is crucial to partner with a bio-logistics provider that can offer the required environment for the shipments to get to their destination securely.
Personnel –
We need to consider the professionals who are supporting the loading, unloading, and handling of the shipments. Key concerns when loading shipments include:
- Whether the accessories, such as the cane or rack, have been pre-conditioned.
- Whether the liquid nitrogen has set the temperature of the dry shipper correctly.
- Whether the dewar has been properly cleansed of any prior spillage.
On the other hand, the professional responsible for the unloading process will need to pay attention if no vapor emits when opening the cryogenic storage dewar. Dry shippers must allow the gaseous liquid nitrogen to escape and prevent safety risks. Obviously, training a professional to securely handle Dry Shippers when carrying fragile contents is essential. Dry shippers look easy-to-use but require trained hands for handling.
Process –
Empty dry shippers weigh more than a regular hand carry shipment. In addition, cryo-shipments are biological in nature and are officially declared in most international airports. These customs regulations and processes across various airports may delay the shipment from timely arriving at its drop-off destination. Some airports may require a pre-filled document during the check-in & departure process, while other airports also require these documents upon arrival. Logistics providers that offer cryo shipping solutions must be knowledgeable on local landscapes and understand the customs procedures for biologic cryo-shipments.
How does Royale’s Dry Shippers mitigate bio-logistics risk?
Royale International’s Cryoshippers are premium quality and IATA certified to ensure biologics are maintained at cryogenic temperatures throughout transit.
/ Packaging designed for biologics products
Our dry shippers can store up to 10L of liquid nitrogen with 10 days of dynamic holding time, accommodating various biological products. Its functional design slows liquid nitrogen loss, retains chemicals with its vacuum materials and repels moisture with its hydrophobic systems. Moreover, Royale’s Cryshippers include accessories to hold various shipment types.
/ Trained and qualified professionals
Through our global Quality Management System (QMS), Royale offers extensive job training to its medical couriers according to our annual training matrix. Furthermore, our experts provide all couriers with loading and unloading guides that include concise instructions when handling shipments. Contact information of dedicated account managers provide an open channel of communication when in need of escalation.
/ Unified and distributed processes
Finally, Royale’s global operating procedures ensure that all Cryoshippers dispatch the same way regardless of the professional handling the shipment. In addition, Royale International has 43 global offices and leverages its dedicated account managers to understand various local logistics regulations. Industry experts file, update and easily distribute customs documents to make a seamless transit.
———— Read about how our Dry Shipper supported a lifesaving transplant ———––
How does Royale commit to premium cryo-shipping services?
Royale International mitigates temperature-controlled logistics risks through its global Quality Mangement System (QMS) accredited under WHO GDP and ISO guidelines. Ultimately, our experts consolidate temperature-controlled shipment requirements and create bespoke and integrated solutions through our global network. We offer a one-stop logistics solution for the advancement of healthcare, pharmaceuticals and biotechnology breakthroughs. Discuss with our industry experts below.
Ask about our temperature-controlled packaging solutions? Contact our industry experts: lifesciences@royaleinternational.com or lifesaving@royaleinternational.com
Looking to join our global courier network?


