Clinical trial – Managing your global supply chain

Drug development has evolved into large-scale multinational clinical research today, involving large pools of potential participants across multiple regions to get FDA’s approval. However, conducting a clinical trial across various locations might lead to serious logistics challenges. Careful planning is required to overcome barriers and bring innovative medical treatments to markets worldwide. As a Life Sciences logistics expert, we have put together a list of ways to manage global clinical trial supply & logistics.
Plan your logistics ahead
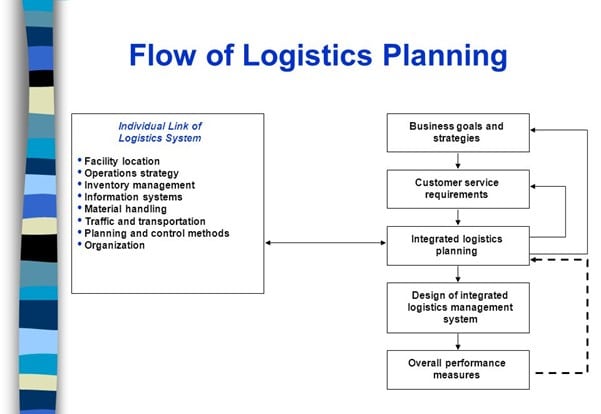
The main aspect of developing efficient clinical trial logistics management is having the right planning. From obtaining goods to planning deliveries from origin and destination and taking care of product storage in a specific temperature range, all these transportation needs have to be fully planned and supported. Planning simplifies the transportation procedures, ensuring a smooth, streamlined supply chain. Eventually, this reduces the impact on company’s productivity and profit. Below you can find the 5-step flow of logistics planning:
Navigating customs regulations
When handling global clinical trials, logistics providers and pharma companies must be aware of the import/export licenses and documents required for each country. As customs vary from country to country, it is essential that your logistics provider keeps track of the latest customs regulations and complies with them throughout the logistics process.
Click here to learn how we ensure the right paperwork for your Life Sciences shipment!
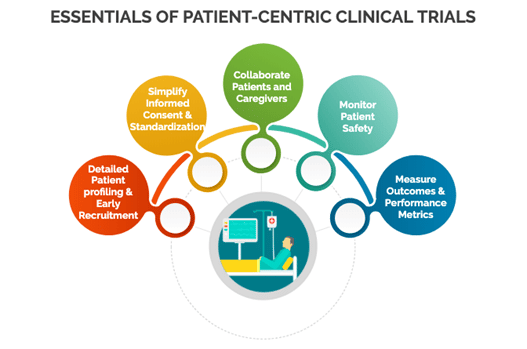
Evaluating patient care models
In global clinical trials, patient care models in terms of patient profiling, safety, consent & compliance with protocol vary significantly across regions. This requires careful planning in patient treatments for each country and its means of transport. Eventually, this improves regional patient retention, reduces time and maintains diagnostic accuracy.
Closely monitor every step shipment milestone
Proactive monitoring of goods and providing updates is extremely important. As your Life Sciences shipment has to go through various checkpoints such as check-ins, security screening, and shipment clearance. Make sure you keep a close eye on each stage in the clinical trial supply chain to deliver the right products to the correct international locations, to meet the SIV date for each clinical trial.
How Royale addresses clinical trial supply chain challenges?
Royale International is a well-reputed organisation that specializes in the worldwide handling and transportation of clinical trials.
Offering customised Life Sciences solutions
Every clinical trial is unique. As an experienced Life Sciences specialist, Royale offers customised clinical trial logistics solutions to fit your business requirements. Moreover, we are able to facilitate your clinical product supply chain needs across the globe, while maintaining high pharmaceutical standards.
Customs documentation
Ranging from customs clearance to import / export permits and product verification reports, all documents that we process fulfil the requirements of the Customs Authority, International Air Transport Association (IATA), and Good Distribution Practice (GDP).
Providing temperature-controlled packaging
In terms of packaging, we provide high-end packaging with the right type, size, material, and validation time, ensuring your products get delivered safely to the destination.
Have a look at a variety of packaging solutions that we offer!
Please click here to view our global offices!


