Shipping with Dry Ice

From medical supplies to vaccines, clinical trials and frozen food. Dry ice shipping is essential to maintain the right temperature and ensure product integrity.
The Concept of Dry Ice Shipping
Dry Ice refers to a solid form of Carbon Dioxide (CO2), which comes in pellets, flakes or blocks. It is usually stored at –78.5°C. Through evaporation, dry ice directly converts from solid texture to gas, which makes it one of the most suitable materials to insulate and protect temperature-controlled products. To transport Life Sciences products with dry ice properly. It is important to have a Life Sciences logistics provider that provides the right packaging and has the correct handling and storing procedures.
How Royale Supports Dry Ice Shipments?
Packaging material for Dry Ice Shipments
When shipping Life Sciences products with dry ice, Royale ensures your contents are at the optimal temperature with a temperature logger before packing. Next, we use a high-quality, strong BioTherm box to maintain a fixed position and ensure product integrity. As dry ice releases CO2 gas continuously, this can build up enough pressure to rupture the packaging. Therefore, the packaging that we use not only allows the release of gas, but also prevents the build-up pressure and slows down the sublimation process (when the substance changes from solid to gas).
Furthermore, we calculate the right amount of dry ice needed based on the packaging and transit time. Next, we carefully assemble the packaging by placing insulating foam (EPS), dry ice and the temperature data logger into the BioTherm box accordingly. To prevent temperature excursions, you must execute these steps carefully.
Click here to have an overview of our packaging solutions.
Labelling for your Package
In most cases, you must affix a Class 9 label to a shipment containing dry ice. To smoothen the logistics & customs clearance process. Royale will attach the Class 9 label to the packaging and list out all key information such as the UN number, the shipping name, the net weight of the dry ice in kilograms, and the shipper’s and consignee’s names and addresses.
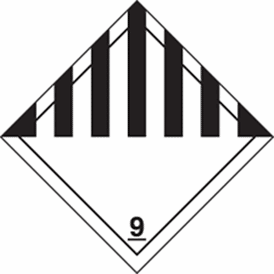
Below is an example of how the information should be listed on the package:
- The UN number: 1845
- The proper shipping name: Dry Ice/ CO2 Solid
- The net weight of the dry ice per package: 4kg
- Information of the shipper
Name: David Roberts
Address: Princess Margaret Hospital, Singapore - Information of the receiver
Name: Anna Hawes
Address: Queen’s Medical Center, New York, United States
Shipping requirements and documentation may vary between countries and carriers. Please contact our Life Sciences expert for more detailed information.
Handling Dry Ice
Royale takes “safety” as a priority. As a leading Life Sciences logistics company, we are extremely careful in handling and delivering dry ice shipments. When handling dry ice, our specialists wear a face shield and goggles to protect their eyes, and a laboratory coat and thermal gloves to protect their skin from splashes.
* Please note that anyone in the supply chain handling dry ice, is required to receive the appropriate level of Life Sciences training.
Click here to view a detailed version of Dry Ice Handling Procedures.
Documentation for your Dry Ice Shipment
Dry ice shipments require a lot of documentation. From the Airway Bill, Export License, Letter of Assurance, and Material Safety Data Sheet to ensure passage through the customs clearance process.
When it comes to dry ice shipping, the packaging, handling procedures and storing process are of critical importance. To overcome these challenges, Royale International has developed a Life Sciences Division that specializes in the worldwide handling and transportation of dry ice shipments. In terms of packaging, we provide high-quality packaging with the right material, validation time, and size, ensuring your product is safely delivered to its destination. Ranging from customs clearance to import / export permits and product verification reports, all documents that we process fulfil the requirements of the Customs Authority, International Air Transport Association (IATA), and Good Distribution Practice (GDP).
Click here to learn more about our Life Sciences services!


